- news
- 04-04-2023
The intricate architecture of collagen is a key contributor to the mechanical properties of diverse tissues, and is of paramount importance in developmental and cancer biology. However, imaging collagen network architecture has proven notoriously challenging. Here, the Milinkovitch lab introduces a simple and robust method of whole-mount staining with ‘Fast Green’ dye that provides unmatched visualisation of 3D collagen network architecture, via confocal or light-sheet microscopy, and is compatible with solvent-based tissue clearing and immunostaining.
In a new publication, Grigorii Timin and Michel Milinkovtich present a very simple and robust novel method of highly-specific whole-mount staining of collagen fibres, providing unmatched visualisation of collagen 3D network architecture in diverse tissue samples.This new approach is compatible with solvent-based tissue clearing and immunostaining. The stunning image that won the first place prize of the 48th annual Nikon Small World Photomicrography Competition was obtained using that technique.
Collagen, the principal structural protein of the extracellular matrix, is the most abundant protein in animals, amounting to approximately one third of the total protein mass of vertebrates. Collagen fibrils can organise into so-called ‘fibres’ that are visible with optical microscopy. Various collagen network architectures endow different tissues with distinctive mechanical properties such as anisotropic stiffness and specific non-linear strain-stiffening responses. Through cell-ECM interactions, collagen architecture also modulates cell behaviours such as cell migration along aligned collagen fibres in developing tissues. Note that the tangential disposition of straightened collagen fibres is considered a signature of certain tumour ECM, whereas perpendicular alignment of collagen fibres in contact with the tumour provides a route for invasion of cancer cells away from the primary cluster. These signatures are often included among the parameters used to predict cancer progression, invasiveness and treatment response.
Given the importance of collagen architecture in developmental and cancer biology, transmission electron microscopy and histological techniques have been widely used for over half a century to visualise collagen in tissues. However, given that the global 3D architecture of the collagen network can typically vary extensively across a given tissue sample, it cannot be inferred effectively from these 2D approaches.
Capitalising on the fluorescence of the dye ‘Fast Green’, the Swiss researchers report a very simple and robust new method of highly-specific whole-mount staining of collagen fibres, providing unmatched visualisation of 3D collagen network architecture via confocal and/or light-sheet microscopy.
Much additional details are available in the original publication:
High-Resolution Confocal and Light-Sheet Imaging of Collagen 3D Network Architecture in Very Large Samples
Grigorii Timin & Michel C. Milinkovitch
iScience 26, 106452
https://doi.org/10.1016/j.isci.2023.106452
Full-resolution images used for Figures 1 to 7 of the original publication are available at https://www.lanevol.org/projects/FGstaining.
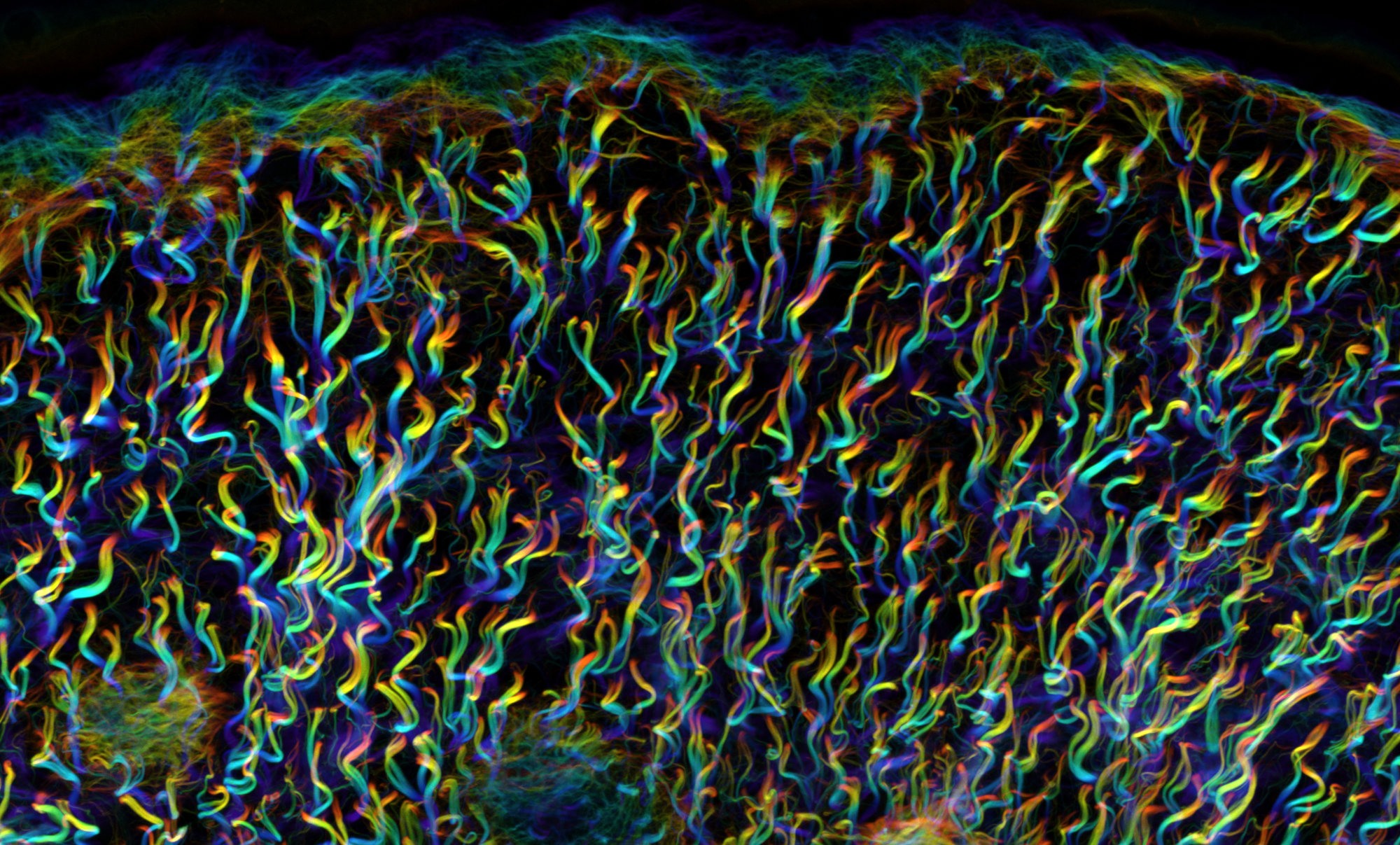
Figure 1: One key advantage of Fast-Green (FG) labelling over the previous state-of-the-art (Second Harmonic Generation, SHG) is that FG allows for collagen network 3D imaging in highly heterogeneous samples. This is explained by the fact that collagen fibres oriented parallel to the direction of laser light propagation are efficiently visualised with FG labelling whereas they do not produce SHG signal. Hence, fibre continuity is correctly identified only with FG staining. The superiority of FG over SHG becomes even more drastic with smaller fibres as they become virtually undetectable by SHG, whereas they are clearly identified with FG labelling.
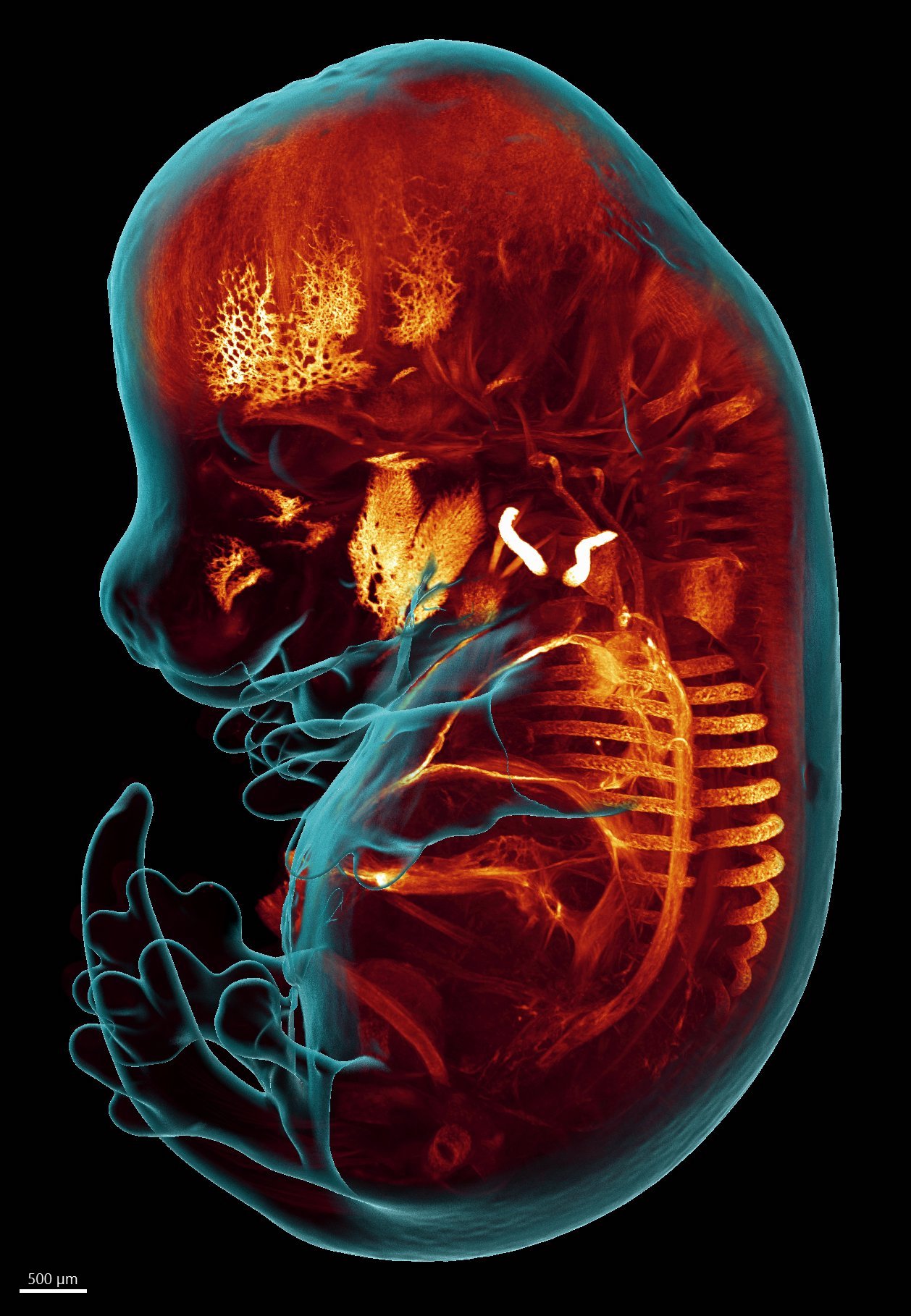
Figures 2: Light-sheet microscopy imaging of an embryonic day 14.5 (E14.5) mouse embryo shows that Fast-Green staining counterstains embryonic structures and organs in a more distinguishable way that nuclear staining. A whole-embryo maximum-intensity projection image shows structures with highest FG accumulation, such as calcified collar bones and skull bones as well as the largest blood vessels.
